
Fat and thin people - both bodies are the result of the action of acetyl CoA.
The A stood for acetyl, since one of CoA's main jobs is to transfer two-carbon units in the form of acetyl between various biological molecules. It can be thought of as the body's 'delivery truck', since it transports its cargo of C2 along the roadways of blood vessels to the retail stores (muscles) where it's unloaded.
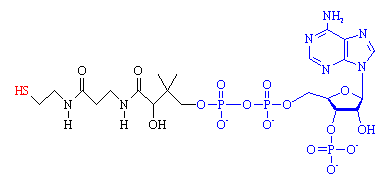
Coenzyme A. The 'business end', the -SH group where the acetyl group attaches is shown in red. The fragment from ADP is shown in blue.
CoA is composed of two main parts, a long protein-like chain (shown in black in the figure), joined to adenosine diphosphate, ADP, (shown in blue) which is one of the molecules (along with ATP) used for energy storage. The important part of the molecule is at the end of the protein chain, which terminates in a sulph-hydryl (-SH) group (red). This group is highly reactive, and links to carboxylic acid molecules via a thioester bond. The most important acid is acetic acid, and when it is joined to CoA, the resulting compound is known as acetyl-CoA.
The thioester link, however, is very high energy bond, and therefore unstable. This means that the acetyl group can be easily transferred to other molecules, and so acetyl-CoA is used as a universal intermediate which provides the C2 fragment for numerous biochemical syntheses.
Fatty acid metabolism
Ketosis gives sweet smelling breathIn animals, both sugars (carbohydrates) and fats can be metabolised to produce energy, and acetyl-CoA is central to keeping the balance between these two. If the body takes in more sugar or fat than it needs, the excess is stored as fat, as described above. But when the body requires this energy again, the fat is metabolised with the help of acetyl-CoA. The stored triglycerides are cleaved to give 3 fatty acid chains and 1 glycerol molecule in a process called lipolysis. The glycerol is converted to glucose, and gives cells energy. And the 3 fatty acids provide an extra source of energy, as the long chains are cleaved 2-carbons at a time to form acetyl-CoA, which can then be fed into the Citric Acid Cycle. In cases of starvation, or where the person has a low carbohydrate diet (such as the Atkins Diet), this process can occur to excess. This can produce an unusually large amount of ketones from the breakdown of fatty acids to acetyl groups. In this situation, the person is said to be suffering from ketosis, and the ketones are excreted in the breath (and urine) giving the person's breath a characteristic sweet, fruity smell, that has been likened to the smell of nail varnish remover (acetone) or sometimes pear drops (ethyl ethanoate).
Fatty acid synthesis (...and the carbons went in two-by-two)
If the C2 unit is not to be oxidised to CO2 and the energy used immediately, another possibility is that it can be used to build important biological molecules. Acetyl-CoA is the starting point for the synthesis of fatty acids from carbohydrates. Fatty acids have carboxylic acid groups (-COOH) at one end, and a long alkane/alkene chain at the other. Examples include linoleic acid (a constituent of margarine), palmitic acid (from palm trees, and used as a constituent of napalm in WW2) and butyric acid (found in butter). Three of these fatty acids then join together with one molecule of glycerol to make triglyceride (also known as triacylglycerol) - and this forms the main constituent of vegetable oil and animal fats. The three fatty acids RCOOH, R'COOH and R"COOH can be all different, all the same, or only two the same, but what they have in common is that they were all synthesised using acetyl-CoA.
See the full explanation of Acetyl Coenzyme A at Bristol University's page